Optimizing experimental conditions
Tuulia Hyotylainen, VTT Technical Research Centre of Finland, FinlandAbstract This article was written by Pavel Jandera, together with Tuulia Hyötyläinen.
In optimization of an LCxLC methodology, the system can be considered to consist of three parts, namely:
1. first dimension separation
2. the sampling of the first dimension
3. the second dimension separation
The optimization should be started with selection of the two columns and the separation mode and then by optimization of the second dimension separation.
LevelBasic
The optimization should be started with:
- Selection of the two columns
- The separation mode
- Optimization of the second dimension separation.
- The speed of the second
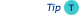 dimension is a key feature of successful separation and the separation should be as fast as possible, with sufficient resolution.
dimension is a key feature of successful separation and the separation should be as fast as possible, with sufficient resolution. - The first dimension
 separation can then be optimized, with the flow rate optimized taking into consideration the modulation time.
separation can then be optimized, with the flow rate optimized taking into consideration the modulation time.
Selection of columns
In the selection of columns, the mode of separation must be selected first. The choice is of course dependent on the type of the sample.
- The separation mechanisms used in the two dimensions of a two-dimensional separation process must have different selectivities for the sample components.
- The sample components should be spread over as broad separation space as possible in both separation dimensions, as shown in the figure.
- Best separation is achieved when the separations are orthogonal, which means that there is no relationship between the retention data of the components of the sample in the two separations.
LCxLC separation, in which sample components are evenly spread across the two dimensions (Horiz. = 1st; Vertical = 2nd)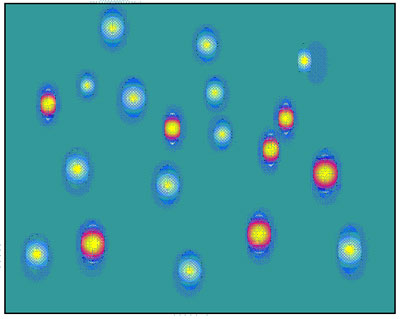
Next, the dimensions of the columns must be considered.
- First dimension. In
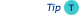 selection of the column configuration, the column with a higher peak capacity should be used in the first dimension.
selection of the column configuration, the column with a higher peak capacity should be used in the first dimension. - Second dimension. The ID of the
 second column should be equal to — or larger than —that of the first column. Short columns packed with small particles (3 μm or less), non porous, superficially porous or monolithic columns are especially suitable for this purpose.
second column should be equal to — or larger than —that of the first column. Short columns packed with small particles (3 μm or less), non porous, superficially porous or monolithic columns are especially suitable for this purpose.
The selection of the mobile phases in the first and in the ![]() second dimensions is very important, because the whole stationary/mobile phase system controls the retention and the separation selectivity.
second dimensions is very important, because the whole stationary/mobile phase system controls the retention and the separation selectivity.
Of course, the mobile phases used in the two dimensions should be compatible.
Isocratic vs. gradient elution
In the first dimension separation, gradient ![]() elution is generally used to obtain high peak capacity (link here to peak capacity definition). Temperature programming offers another possibility for increasing peak capacity, especially in the separation of macromolecular or oligomer compounds.
elution is generally used to obtain high peak capacity (link here to peak capacity definition). Temperature programming offers another possibility for increasing peak capacity, especially in the separation of macromolecular or oligomer compounds.
It is possible to use parallel gradients spanning over the whole gradient time both in the first and in the second dimension. If the second-dimension gradient run over the whole 2D separation time is shallow enough, the separation conditions are quasi-![]() isocratic and do not cause problems in the evaluation of the 2D data.
isocratic and do not cause problems in the evaluation of the 2D data.
Selecting Columns and Conditions
The crucial step in the ![]() development of a 2D LC×LC separation is the selection of the first- and second-dimension separation systems. (here comes a link to more information).
development of a 2D LC×LC separation is the selection of the first- and second-dimension separation systems. (here comes a link to more information).
Various combinations of RP and NP, ion-exchange (IEC), or size-exclusion (SEC) LC modes can be utilized in LCxLC. Selected examples are shown in the tables. In these combinations, the separation is based on the differences in the molecular structure of separated compounds, such as size, polarity and shape, or the specific charge of ionic ![]() compounds. The retention in ion-exchange systems is generally controlled by electrostatic ion-exchange interactions, but can be more or less affected by non-ionic interactions with the stationary phase matrix.
compounds. The retention in ion-exchange systems is generally controlled by electrostatic ion-exchange interactions, but can be more or less affected by non-ionic interactions with the stationary phase matrix.
In practice, the separation selectivities in different systems can be more or less correlated, due to the mixed character of the stationary phase and mobile phase interactions. This must be taken into account when developing real world LC×LC separations.
Since the mobile phases used in RP×RP, IEC×RP, RP×SEC or NP×SEC systems are usually fully miscible and have similar physicochemical properties, these are in practice the most commonly used combinations:
- For example, combinations of IEC-LC and RPLC with either salt concentrations or pH gradients can be used for separations of ionic compounds, acids or bases.
- The combination of SEC with either NPLC or RPLC is useful for separations of macromolecules, such as synthetic polymers and biopolymers.
- An NPLC×RPLC combination is more demanding, due to eluent incompatibility, and is useful for separations of samples with two or more types of structural elements differing in lipophilic and polar properties. Table x lists typical combinations for LCxLC.
NPLCxRPLC and RPLCx NPLC
Separations in RP and NP systems utilize the differences in the polarities and lipophilities of the analytes, but the size of the molecules and their acidity/basicity also contribute more or less to the retention in the RP and NP systems. In most cases, NPLC is used for the first dimension separation and RPLC for the second dimension separation.
A combination of RP and NP systems can be used for 2D separations of samples with two or more types of structural elements differing in lipophilic and polar properties:
- In RPLC, a constant increase in retention often occurs with a regular increase in the number of lipophilic repeat units (e.g. methylene groups),
- whereas polar repeat units cause a regular increase in log k in NP systems.
- Successful selection of 2D systems requires effective use of selective interactions between the analytes and the stationary and the mobile phases in different RP and NP separation systems.
The compatibility of mobile phases in the first and in the second dimension is very important in LCxLC. The transfer is usually feasible from an NP to a non-aqueous or high-organic RP system.
- When transferring the organic solvent NP fractions to
 highly aqueous RP second dimension, the compatibility problems can be solved by removing organic solvents by evaporation in a sample-transfer loop evacuated under high temperature.
highly aqueous RP second dimension, the compatibility problems can be solved by removing organic solvents by evaporation in a sample-transfer loop evacuated under high temperature. - On-line transfer from an RP to an NP system is more difficult, because water contained in the fractions transferred from the RP system may deactivate the polar stationary phase and destroy the separation in the second, normal-phase dimension. This problem is most serious with bare silica NP stationary phase.
- Non-aqueous RP with NP compatible mobile
 phases can be used either in the first or in the second dimension, if the sample compounds are lipophilic.
phases can be used either in the first or in the second dimension, if the sample compounds are lipophilic.
With polar sample compounds, hydrophilic interaction chromatography (HILIC) can be used in the second dimension, either on bare silica or less polar stationary phases, for example aminopropyl-, cyano-, diol-, or carbamoyl- derivatized silica (TSK gel amide) columns, or specific bonded stationary phases, such as poly(2-sulphoethyl) aspartamide, or a sulfobetaine zwitterionic ZIC-HILIC column, which allow the use of mobile phases containing up to 70% water, even though mobile phase more rich in mobile phases are used more frequently, as the retention of polar compounds in HILIC strongly decreases with increasing concentration of water in the mobile phase.
RPLCxRPLC, RPLC-IEX
LC×LC combinations of RPLC and ion-exchange (IEX) LC, with either salt concentration or pH gradients, are often used for separations of ionic compounds, acids or bases.
Mobile phases used in RPLC×RPLC and RPLC×IEC separation systems are usually fully miscible and have similar physicochemical properties. However, mobile phases with great differences in viscosities (such as acetonitrile–water and methanol–water) should be avoided, otherwise flow instabilities may occur, resulting in considerable broadening or distortion of the peak shape of compounds separated in the second dimension.
In optimization of RPLC×RPLC or IEC×RPLC it should be noted that too high concentration of organic solvent in the first-dimension mobile phase may cause problems because such solvent has too high elution strength in the second dimension RP system ![]() and as a result, peaks are broadened in the second dimension separation.
and as a result, peaks are broadened in the second dimension separation.
RPLC×RPLC systems have been applied for the analysis of complex food, natural extracts, pharmaceutical and environmental samples.
RPLC×IEX and SEC×IEX
The retention in IEC is controlled principally by the solute charge, so that the IEX separation mechanism differs from the SEC and RPLC. However, neither RP×IEX nor SEC×IEX systems are fully orthogonal, presumably because of hydrophobic interactions with the non-polar moieties in the ion exchange stationary phases.
- Combinations of ion-exchange (solvent or pH gradient) separations in the first dimension and RP separation in the second dimensions are useful for LC×LC of weakly acidic or basic compounds.
- For RPLC×IEX separations of peptides and proteins or biological amines, 2D systems employing an anion-exchange (SAX) or a cation-exchange (SCX) column with a salt concentration gradient (step or continuous) in the first dimension and a RP column in the second dimension can be used.
- RP×SCX systems are most easy to use with increasing ionic strength gradients to elute peptides from the first dimension SCX column and transfer it onto the second RP dimension. Some hydrophobic peptides may be incompletely recovered or elute with poor peak shape from the first-dimension column as a result of non-specific interactions with the matrix of ion exchangers. The elution can be enhanced by adding organic solvents to the mobile phase, but the aqueous–organic solvent decreases the retention of peptides on the 2D RP column, which may affect the on-line fraction transfer to the second dimension.
- Proteins can be separated according to their isoelectric points on an ion-exchange column using pH gradients in the first dimension and according to their hydrophobicity on an RP column in the second dimension.
SEC×NPLC and SEC×RPLC
SEC is often combined with NP or RP for 2D separations of synthetic polymers and biopolymers to provide separations based on the molar mass distribution (MMD) in one dimension and on the polarity (or lipophility) of the end groups in the other.
- Conventional SEC columns are usually used in the first dimension as they are relatively long and the SEC analysis requires relatively long time, whereas an NP (or RP) column is used in the second dimension for separation of heart-cut fractions from the SEC column.
- Comprehensive NPLC×SEC or RPLC×SEC using short SEC columns (50 mm or less) for fast separations in the second dimension and a longer NP or RP column in the first dimension may be used to improve the separation of polymers according to the chemical composition (end group) distribution.
2D separations of polymers often combine size-exclusion chromatography (SEC) for the separation according to the distribution of molar masses in one dimension and normal-phase, aqueous–organic or non-aqueous RPLC systems (depending on the polarity of the sample) in the other dimension under "near-critical" conditions.
SEC×NPLC has been applied mostly to the separation and characterization of synthetic polymers, copolymers or polymer blends that are soluble in organic solvents.
- In the NPLC, usually a silica gel or cyanopropyl-modified silica column is used with tetrahydrofuran–cyclohexane, dichoromethane–heptane, dichloromethane–acetonitrile, dichloromethane–methanol, trichloromethane–cyclohexane, and so on, mixed mobile phases, either with isocratic or with gradient elution,
- THF is most frequently used as the SEC mobile phase.
- Proteins, peptide fragments, or other biopolymers are often separated by SEC in the first dimension and RP chromatography in the second dimension.
How to evaluate the separation selectivity
There are several ways to evaluate the separation selectivity. The easiest way is visual evaluation of how the peak pairs are scattered on the plot. It is also possible to calculate the normalized retention times (or retention factors) for the target analytes in both columns, determining the correlations between the values obtained for both columns. It should be noted that the correlation coefficients should be always evaluated together with the fraction of the 2D-separation space, which is occupied by chromatographic peaks.




