LIMS
Rick Slikkerveer, ITC Validation Consultants, The NetherlandsAbstract A laboratory deals with many projects. Many standard and non-standard tests are carried out on hundreds of different samples by a great number of analysts using different analytical instruments, analytical methods and chemicals.
A laboratory generates thousands of ‘certificates of analysis’, management reports and invoices.
A Laboratory Information Management System (LIMS) is an excellent tool to support the laboratory enabling it to manage the processes described above. This chapter describes the essential aspects of a successful LIMS system.
LevelBasic
A laboratory is a complex organisation and therefore is also complex to manage. It must deal with many projects and hundreds of different samples simultaneously. Many hundreds of standard and non-standard tests are carried out by a great number of analysts using ![]() different analytical instruments, analytical methods and chemicals.
different analytical instruments, analytical methods and chemicals.
Typically a laboratory generates thousands of ‘![]() certificates of analysis’, management reports and invoices. It has been calculated that the administrative work is about 15% of the total effort.
certificates of analysis’, management reports and invoices. It has been calculated that the administrative work is about 15% of the total effort.
A Laboratory Information Management System (LIMS) is an excellent tool to support the laboratory enabling it to manage the processes described above and as such to reduce the administrative workload.
After the introduction in the early eighties, thousands of laboratories worldwide have installed LIMS. LIMS implementations vary from small laboratories with simple requirements to large multi-site laboratories with complex requirements. Since LIMS systems help to manage laboratory processes in different types of laboratories, they must have different functionalities to suit the different ![]() domains. Another prerequisite for LIMS systems is that they must be very flexible and easy to configure. When purchasing a LIMS, this requisite shall be tested in advance.
domains. Another prerequisite for LIMS systems is that they must be very flexible and easy to configure. When purchasing a LIMS, this requisite shall be tested in advance.
LIMS Concept Model
The concept model below represents the functionality of a LIMS.
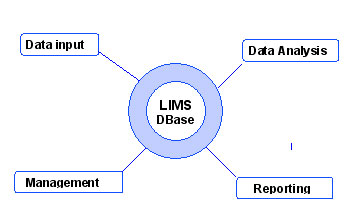
The central element of LIMS is the computer ![]() database. It is the central hub for all LIMS interactions. It is designed to systematically manage laboratory data.
database. It is the central hub for all LIMS interactions. It is designed to systematically manage laboratory data.
The database has four major satellite functional components:
- Data and information input.
This function manages the communication of data and information to and from LIMS.
manages the communication of data and information to and from LIMS. - Data analysis.
Data analysis is the process of verifying, manipulating, transforming, and displaying existing database information.
process of verifying, manipulating, transforming, and displaying existing database information. - Workflow management.
The management function is based on the inner LIMS hierarchy: Submission – Samples – Tests – Results. LIMS monitors the progress of each submission, sample, task and result by adding a date/time stamp status during the analytical process. Some values are updated by LIMS when certain milestones are passed, others manually.
progress of each submission, sample, task and result by adding a date/time stamp status during the analytical process. Some values are updated by LIMS when certain milestones are passed, others manually. - Data and information reporting.
Reporting makes it possible to extract, organise and present information which is stored in LIMS. Pre-defined or ad hoc queries are used. Reports can be either hard copies or in electronic format.
present information which is stored in LIMS. Pre-defined or ad hoc queries are used. Reports can be either hard copies or in electronic format.
Compliance
Compliance with US - 21CFR Part 11 and EU - Annex 11
When LIMS is deployed in a GXP regulated environment it must comply with EU and/or US Federal Legislation. EU – Annex 11 (draft April, 2008) and US – 21CFR Part 11 with respect to electronic records (E-records) and electronic signatures (E-signatures). Both legislations require a number of functional elements to be built in LIMS. From a Business standpoint it is obvious that these elements are important in a non-regulated environment also.
Elements included are:
- Traceability
- Electronic signature
- Automated electronic record-keeping function
- Stringent access rules, password security
- Document and data change controls
- Audit trail
- Version control and data integrity by not allowing changes to critical information such as test plans, specifications, test methods and other controlled procedures.
LIMS work flow
LIMS manages the flow of data and information in a predefined fashion. A laboratory can be regarded as a system with input and output of data and information. The purpose of the workflow diagram is to map the laboratory work flow (processing of samples) into LIMS functions and interaction points.
| Request | The process starts with a request for analysis and the samples. | |
| Planning | LIMS can be programmed to automatically schedule work. | |
| Analysis | After measurement results of assays must be entered in LIMS automatically or manually. | |
| Validation | When all results have been entered, the request can be reviewed and approved by the appropriate person. | |
| Report | Once test results are verified/validated, they can be reported to the customer. | |
| Archiving | Approved requests can be stored in the database as long as defined in the quality management system. |
Work flow in detail
| Output | ||
| Submitter information |
| Details | ||
| Worklist: | - By analyst - By technique - By test - By sample | |
|
Workload list by unit
| - Analyst - Instrument - Method SOP - Reagents |
| Input | Details | |
| Raw data with: | - Sample code - Analyst - Method - Instrument - Date | |
| Final result | Results of assays can be entered in LIMS |
| Output | Details | |
| All data and information | - By sample - By test - By request | |
| Results from |
| Output | Details | |
| Certificate of analysis | - By sample - By request | |
| Management information | ||
| Control charts | ||
| Invoicing |
| Output | Details | |
| All data and information | - By sample - By submission | |
| Full history |
Laboratory Management
By collecting statistics and ![]() time-stamps at various points in the process, the management functions can generate reports for the laboratory management.
time-stamps at various points in the process, the management functions can generate reports for the laboratory management.
Miscellaneous Functionality
In their pursuit to automate processes as ![]() much as possible, LIMS suppliers developed extended functionality for LIMS.
much as possible, LIMS suppliers developed extended functionality for LIMS.
Extended use of LIMS
A LIMS was initially developed for an analytical laboratory and the standard analytical process. But after a few years of experience users asked for more enhanced functionality. To meet the specific needs of laboratories and industry, suppliers of LIMS have developed a number of add-on application modules.
A number of important modules are:
Module for Lot or Batch Quality Management
This module has been developed as a management tool to support organisations that manufacture products by Batch or by Lot.
- The module allows for the definition of all the laboratory testing that will occur as a product is being manufactured.
- The module provides a complete view of the quality history of a batch or lot, from raw materials and manufacturing steps to final production and distribution.
- This module is typically developed for pharmaceutical, chemical, petrochemical and food industries.
Module for Stability (Shelf-life) Management
This module has been developed as a management tool to support organisations that perform stability studies (e.g. pharmaceutical industry).
- Stability testing assesses the shelf life of products under different conditions; product package type, temperature, time points, humidity, and other variables.
- The module makes it possible to create, review, approve and initiate a study and automatically scheduling all sampling and testing.
Scheduling Module
This module has been developed to manage routine sampling and testing schedules.
- It allows test samples to be created in LIMS automatically at any date-time interval.
- It may also include the generation of collection worksheets along with sampling points, and sampling instructions.
Added on Software Solution
LIMS vendors offer software solutions providing integration between the laboratory and the manufacturing floor for:
- Enterprise Resource Planning (ERP) applications like
- SAP
- Oracle Applications or Microsoft Dynamics
- Manufacturing Execution Systems (MES) like
- Siemens
- GE Fanuc.
Considerations for purchasing LIMS
Configuration versus customization of ![]() LIMS
LIMS
Configuration means setting up a program or computer system for a particular application. Configurable software package provide standard interfaces and functions enabling configuration of user specific business processes. When a LIMS can not be configured to the customer needs, extensions have to be developed to meet the specific needs of the customer. Customization, however, is time-consuming and very expensive.
Commercially available LIMS have flexible configuration tools that allow system administrators to easily establish and maintain their laboratory’s unique workflow and business rules through ad hoc or pre-defined lifecycles. Nevertheless, each lab has individual ![]() requirements—and each application requires its own configuration, validation and testing cycle.
requirements—and each application requires its own configuration, validation and testing cycle.
When a supplier upgrades its version of LIMS, they usually guarantee upward compatibility. This means that all functionality from the previous version will also work in the new version. However, any customization to the system is the responsibility of the customer and hence the automatically upward compatible. The system owner must make a decision here: Update or not update. Not updating means not benefiting from the latest technologies and its possibilities for the business. Updating would entail to repeating the customization cycle. In the long run that may turn out to be a major ![]() problem.
problem.
Examples of configuration and customization are:
- LIMS in a QC Laboratory
A QC laboratory is a sample oriented environment with predefined samples, tests and specifications. Only samples that do not meet the predefined specifications are important for further management actions.
that do not meet the predefined specifications are important for further management actions. - LIMS in a R&D Laboratory
An R&D laboratory is a project oriented environment where many non-routine tests in unknown samples are performed and where method development is an essential part of the analytical process. In an R&D laboratory data must be further interpreted to obtain relevant information.
further interpreted to obtain relevant information.
Costs and Benefits of a LIMS
Implementing a LIMS will require a significant investment.
- Before deciding to purchase a LIMS an organization would need to prepare a sound business case. It demonstrates the value of a LIMS and, as such, justifies the costs involved.
- A major part of the business case is a cost-benefit analysis. In this analysis, the organization would also want to consider the option of not-implementing LIMS.
- Costs can be defined in two ways: initial costs and ongoing costs. Initial costs include purchasing costs and implementation costs. Ongoing costs are the total costs of ownership (TCO) (licenses, maintenance of hardware, software and data etc.).
- Benefits can be defined in three ways; hard savings, which can be tangible, and soft savings, which may be intangible and hard to quantify.
Part of the quality system
Because LIMS is part of the functionality of the laboratory it must be in compliance with the quality system of the laboratory. Like any other system in the laboratory this means that it must be 'fit for purpose'. Therefore, a LIMS must be validated.
Validation means establishing documented evidence which provides a high degree of assurance that a specific process (i.e. the LIMS) will consistently produce a product meeting its predetermined specifications and quality attributes (FDA definition 1987).
In the case of a LIMS this means that the system shall be purchased, implemented and maintained in a controlled way during the lifetime (lifecycle) of the system (from initiation to retirement).
This will be discussed in a fortcoming article on LIMS. Rick Slikkerveer.






