Abstract Ions separated by IC may lack sufficient UV absorbance so that light absorbance detectors commonly employed in HPLC cannot be used in all cases. Detection based on conductivity measurements is the preferred mode for routine work in IC, although new applications have necessitated the development of advanced detection techniques that might provide increased sensitivity, better selectivity, and more information about the identity, the structure or the elemental composition of the analytes.
The material presented on the next chapters will cover the principles of various detection techniques nowadays used in IC. Although IC includes a variety of separation modes such as ion-exchange, ion-exclusion, ion-pairing, or ion-chelation, the discussion will mainly be restricted to ion-exchange chromatography, which seems to be the currently most widely applied separation mode in IC.
LevelBasic
A characteristic of the ion-exchange process is the fact that during the separation process analyte ions displace co-ions (i.e. the ions of the same charge in the mobile phase). Therefore, when analyte ions pass through the detector, this volume of mobile phase will contain an increased concentration of analyte ions and at the same time a decreased concentration of the co-ions.
The detection signal depends on the difference between a property of the analyte ion and a property of the co-ion (in the worst case, this difference can even be zero). The signal also depends on the number of moles of co-ions displaced by 1 mol of analyte ions (the so-called transfer ratio). This ratio is simply the charge number zA of the analyte ion A divided by the charge number zM of the co-ion M of the mobile phase. For highly charged ions, z is not necessarily the formal charge number but may be lower because for steric reasons not all of the charged functionalities of the ions may be able to interact simultaneously with the stationary phase.
This characteristic behaviour of an ion-exchange chromatographic system should be kept in mind when predicting the response for commonly used detection techniques (see Figure 1).
1. Concentration and Response in IC detection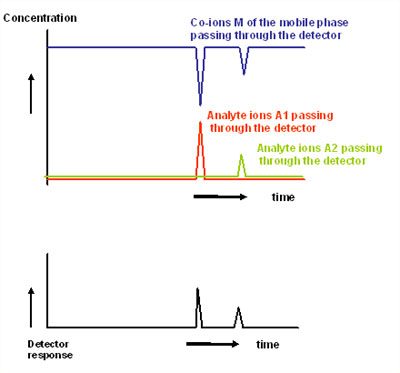
 Did you ever try to explain separation to your employees or students? Well, try no more: Lee Polite did it for you in a way which is hard to beat. We will open up one example of his whiteboard class.
Did you ever try to explain separation to your employees or students? Well, try no more: Lee Polite did it for you in a way which is hard to beat. We will open up one example of his whiteboard class. 




