LevelBasic
A schematic diagram of a split inlet and photographs of the top and bottom of an actual split inlet are shown in Figures 1, 2 and 3.
There are several critical components that can be adjusted.
- At the top or front of the inlet, a septum is used to provide a point for injection using a syringe without disrupting the carrier gas flow and without causing a leak.
- Carrier gas flows into the top of the inlet, directly below the septum.
- At this point, the flow is split between the inlet liner and a septum purge valve. The septum purge serves to provide a small (usually about 3 mL/min) underneath the septum to help keep it clean. The other path goes through the inlet liner. It is here where the syringe needle will deposit the sample.
- In a split injection, there is a large (typically 50-100 mL/min) flow of carrier gas through the inlet liner. Ideally, the sample will be vaporized and mixed with the carrier gas.
- At the end of the inlet liner, there are two possible exits. First is the capillary column, which generally has a low volumetric flow rate (typically about 1 mL/min); next is the split vent exit, which has a high flow rate (typically 50-100 mL/min) which is controlled by a needle valve.
Schematic of split inlet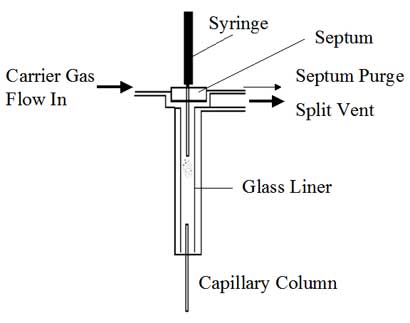 Top of split inlet
Top of split inlet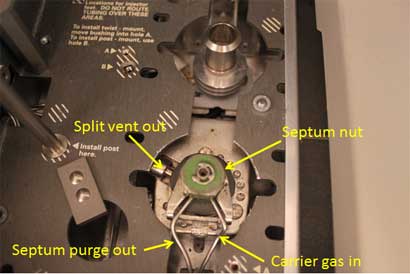
Bottom of split inlet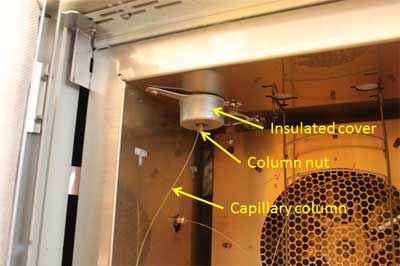
The ratio of flow rate through the split vent to flow rate through the column generates the split ratio, which allows estimation and control of the sample quantity entering the column. For split injection, the inlet is typically back pressure regulated, which provides for a constant flow rate into the column, while the split vent flow is varied.
 Did you ever try to explain separation to your employees or students? Well, try no more: Lee Polite did it for you in a way which is hard to beat. We will open up one example of his whiteboard class.
Did you ever try to explain separation to your employees or students? Well, try no more: Lee Polite did it for you in a way which is hard to beat. We will open up one example of his whiteboard class. 




