Abstract Conductivity detectors register ionic components by measuring the resistance across two electrodes in solution. The presence of ions lowers the resistance, which is inversely proportional to the conductivity. Conductivity detectors are not compatible with high buffer concentrations and require careful control of temperature.
KeywordsConductivity, Buffer ions, Temperature, Resistance, Electrodes
LevelBasic
This detector can detect all ionisable organic and inorganic components. The detector cell contains two stainless steel, gold, or platinum electrodes across which a constant alternating current can be applied. The resistance across the two electrodes (i.e. the resistance of the mobile phase) is measured. As long as the eluent is free of ions, the resistance is high. The presence of ions, however, reduces the resistance since their diffusion can transfer charge between the two electrodes.
Detection of ions with conductivity detection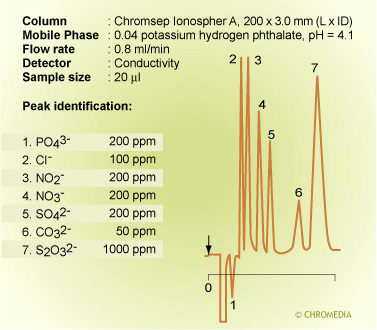
The detector measures resistance, but reports conductivity, which is inversely proportional to the resistance. If the mobile phase contains a buffer, it will give a background signal. This is not a problem at low buffer concentrations, as the background can be compensated electronically. Concentrated buffers should be avoided, however. Since the conductivity of a solution is a function of temperature, modern conductivity detectors employ a thermostatted flow cell and require that the column be thermostatted as well.
 Did you ever try to explain separation to your employees or students? Well, try no more: Lee Polite did it for you in a way which is hard to beat. We will open up one example of his whiteboard class.
Did you ever try to explain separation to your employees or students? Well, try no more: Lee Polite did it for you in a way which is hard to beat. We will open up one example of his whiteboard class. 




