![]() Aluminium oxide adsorbents in GSC have been succesfully applied to the separation of saturated and unsaturated C1 to C10 hydrocarbons. Modification of alumina surfaces has, however proved neccessary to reduce peak tailing. Considerable improvements in the separation efficiency were achieved by modification with water, organic liquids or inorganic salts.
Aluminium oxide adsorbents in GSC have been succesfully applied to the separation of saturated and unsaturated C1 to C10 hydrocarbons. Modification of alumina surfaces has, however proved neccessary to reduce peak tailing. Considerable improvements in the separation efficiency were achieved by modification with water, organic liquids or inorganic salts.
| Aluminum oxide PLOT column features:
|
Retention behavior towards water and impurities
The retention behaviour of alumina is strongly influenced by water. Although the alumina is deactivated with potassium chloride, water can still be adsorbed by the alumina surface, leading to a decrease in retention times.
For high retention time reproducibility, absolutely water-free carrier gas is required. To remove water from the carrier gas, a molecular-sieve-containing moisture filter must be placed in the carrier gas flow-line. If water is present in the sample, it will remain on the column and will consequently reduce the retention factors.
To remove the water from the alumina surface a temperature programmed regeneration to 200°C should be applied. The regeneration will take from a few minutes up to a few hours, depending on the amount of water adsorbed.
Aluminium oxide exhibits very strong retention for all compounds, especially polar compounds like alcohols, aldehydes and ketones. These compounds will not elute from the column, not even at 200°C because their interaction with the active sites on the alumina surface is too strong. As a result, they remain on the column where they can influence retention behaviour. Removal of these compounds is possible only by long-term conditioning or by applying backflushing. For reproducible long-term analysis it is important that concentrations of polar impurities and high-boiling volatile materials are as low as possible.
Applications of alumina PLOT columns
The fused silica Al2O3/KCl PLOT column is a very powerful tool for the analysis of hydrocarbons up to C10. This can be demonstrated in a number of applications. In all cases, the open-tubular PLOT columns are significantly faster than their packed analogues.
Volatile hydrocarbons on packed aluminiumoxide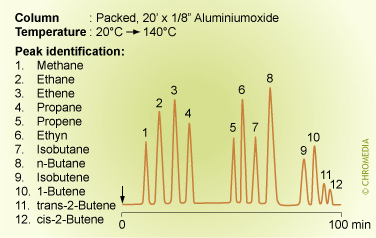
Impurities in 1,3-butadiene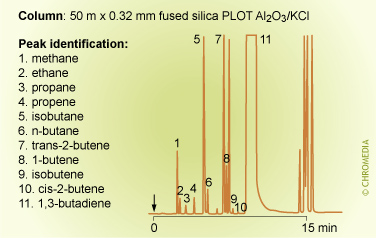
 Did you ever try to explain separation to your employees or students? Well, try no more: Lee Polite did it for you in a way which is hard to beat. We will open up one example of his whiteboard class.
Did you ever try to explain separation to your employees or students? Well, try no more: Lee Polite did it for you in a way which is hard to beat. We will open up one example of his whiteboard class. 




