The thermal conductivity detector (TCD) is a universal detector based on the measurement of the thermal conductivity of a gas. The TCD measures the difference in heat conductivity between pure carrier gas and carrier gas containing sample components.
The core of the TCD is a filament, a thin wire often made of tungsten, platinum or nickel. Since the resistance of this filament is dependent on its temperature, a change in temperature will result in a change in resistance. This change can be detected electronically.
| Detection principle:
|
The detector has the highest sensitivity when the difference between the carrier gas and the sample components is large. This is the case with the lighter carrier gases, such as hydrogen and helium.
It is not recommended to use nitrogen or argon due to the small differences in thermal conductivity, except when hydrogen or helium are to be measured.
For a capillary TCD it is important that the volume of the measuring cell is small. The use of capillary columns in combination with a large ('packed column') TCD would lead to a large loss of efficiency as a result of peak broadening in the detector cell.
Compare the cell volume of a packed TCD with a capillary TCD:
- Packed: 220 nL
- Capillary (medium- bore 0.25/0.32 mm): 10 nL
- Capillary (narrow-bore/high speed GC's; 0.10/ 0.15mm): 20 nL
In fact, a 10 nL cell is too large. Conventional TCD's are therefore equipped with a make-up flow. An addition of about 1 to 10 mL/min extra carrier gas is sufficient to prevent the influence of dead volume. Since a TCD is a concentration-sensitive detector one should take into account a loss of sensitivity when make-up is added.
TCD chromatogram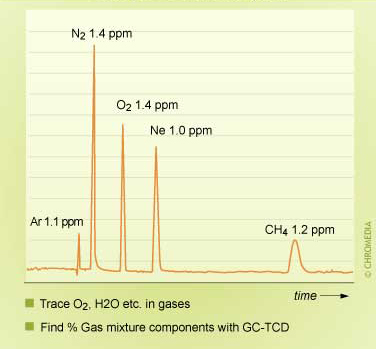
 Did you ever try to explain separation to your employees or students? Well, try no more: Lee Polite did it for you in a way which is hard to beat. We will open up one example of his whiteboard class.
Did you ever try to explain separation to your employees or students? Well, try no more: Lee Polite did it for you in a way which is hard to beat. We will open up one example of his whiteboard class. 




